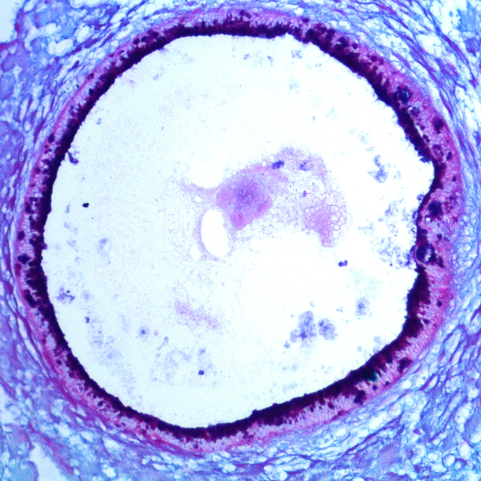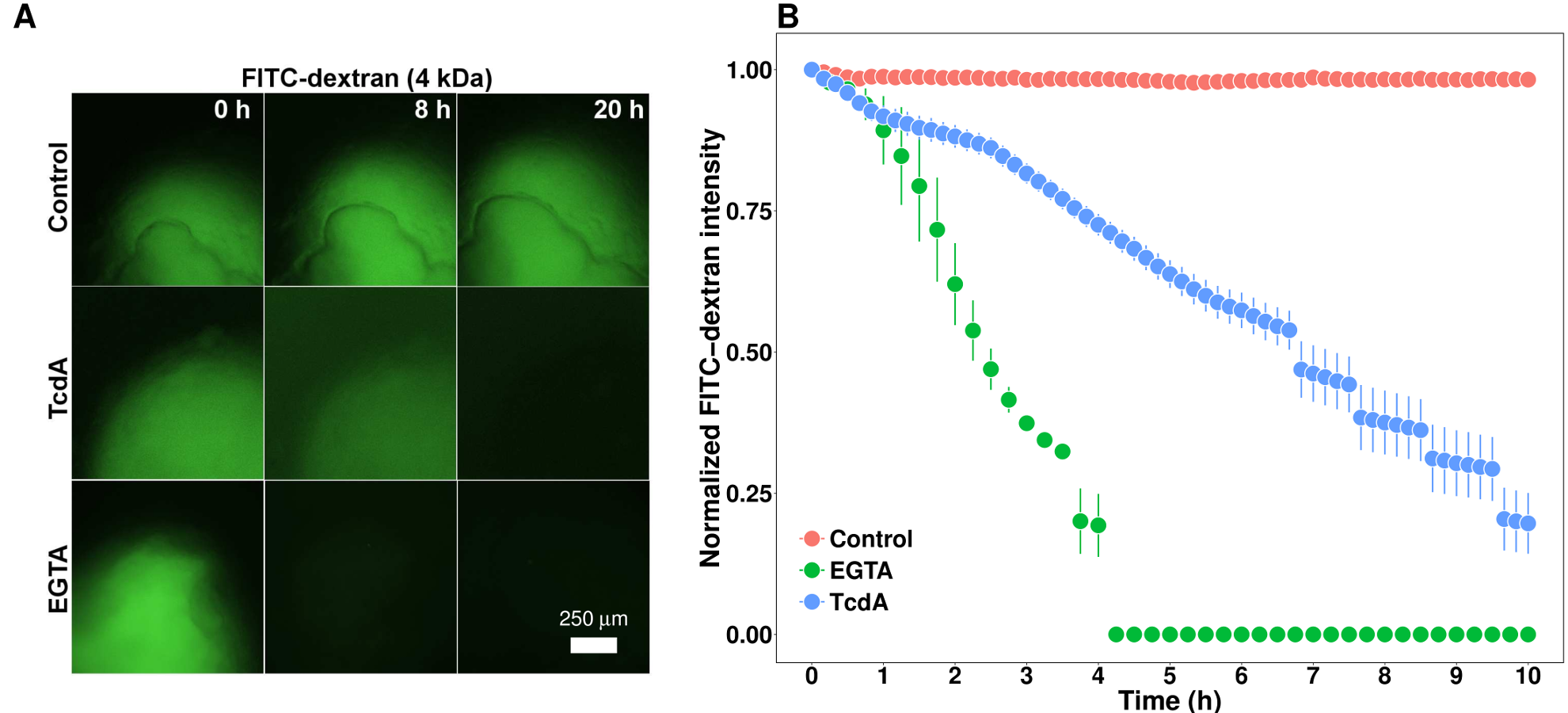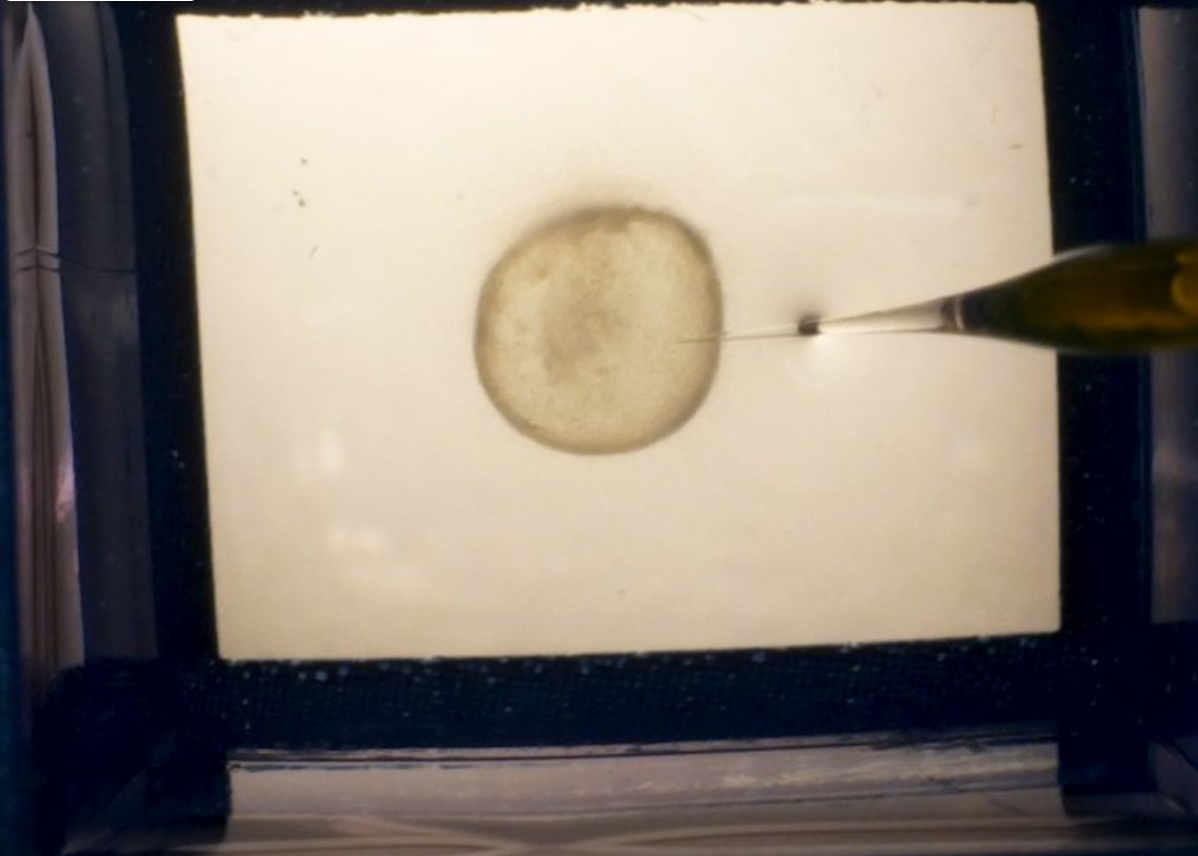
Advances in 3D culture of intestinal tissues obtained through biopsy or generated from pluripotent stem cells via directed differentiation, have resulted in sophisticated in vitro models of the intestinal mucosa. Leveraging these emerging model systems will require adaptation of tools and techniques developed for 2D culture systems and animals. Here, we describe a technique for measuring epithelial barrier permeability in human intestinal organoids in real-time. This is accomplished by microinjection of fluorescently-labeled dextran and imaging on an inverted microscope fitted with epifluorescent filters. Real-time measurement of the barrier permeability in intestinal organoids facilitates the generation of high-resolution temporal data in human intestinal epithelial tissue, although this technique can also be applied to fixed timepoint imaging approaches. This protocol is readily adaptable for the measurement of epithelial barrier permeability following exposure to pharmacologic agents, bacterial products or toxins, or live microorganisms. With minor modifications, this protocol can also serve as a general primer on microinjection of intestinal organoids and users may choose to supplement this protocol with additional or alternative downstream applications following microinjection.
David R. Hill, Sha Huang, Yu-Hwai Tsai, Jason R. Spence, Vincent B. Young
JoVE,
2017
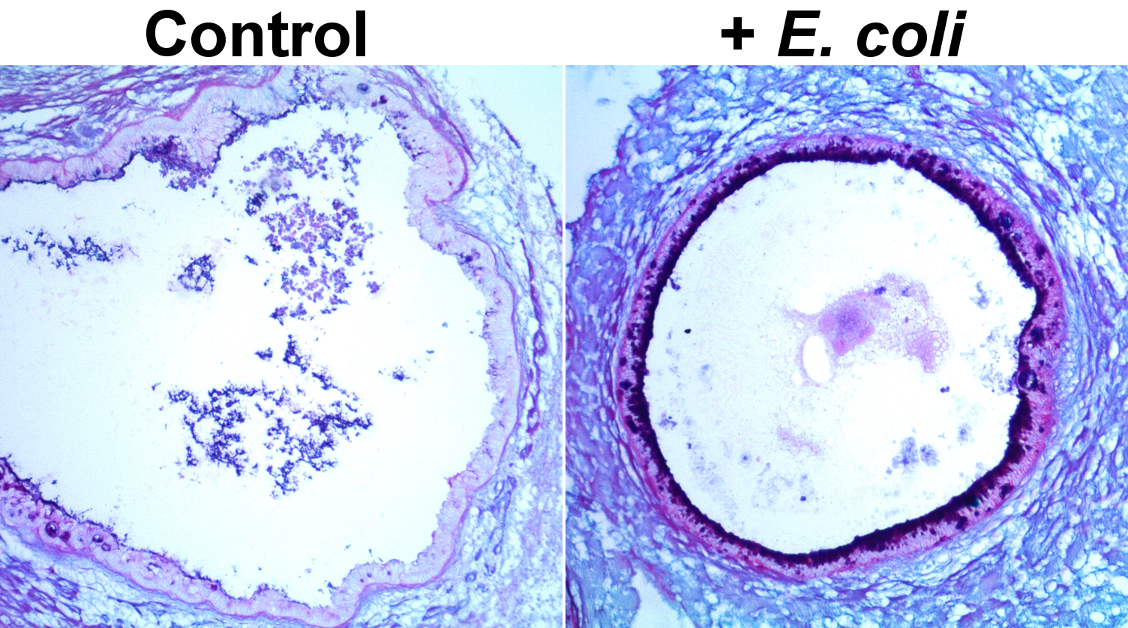
The human gastrointestinal tract is immature at birth, yet must adapt to dramatic changes such as oral nutrition and microbial colonization. The confluence of these factors can lead to severe inflammatory disease in premature infants; however, investigating complex environment-host interactions is difficult due to limited access to immature human tissue. Here, we demonstrate that the epithelium of human pluripotent stem-cell-derived human intestinal organoids is globally similar to the immature human epithelium and we utilize HIOs to investigate complex host-microbe interactions in this naive epithelium. Our findings demonstrate that the immature epithelium is intrinsically capable of establishing a stable host-microbe symbiosis. Microbial colonization leads to complex contact and hypoxia driven responses resulting in increased antimicrobial peptide production, maturation of the mucus layer, and improved barrier function. These studies lay the groundwork for an improved mechanistic understanding of how colonization influences development of the immature human intestine.
David R Hill, Sha Huang, Melinda S Nagy, Veda K Yadagiri, Courtney Fields, Dishari Mukherjee, Brooke Bons, Priya H Dedhia, Alana M Chin, Yu-Hwai Tsai, Shrikar Thodla, Thomas M Schmidt, Seth Walk, Vincent B Young, Jason R Spence
eLife,
2017
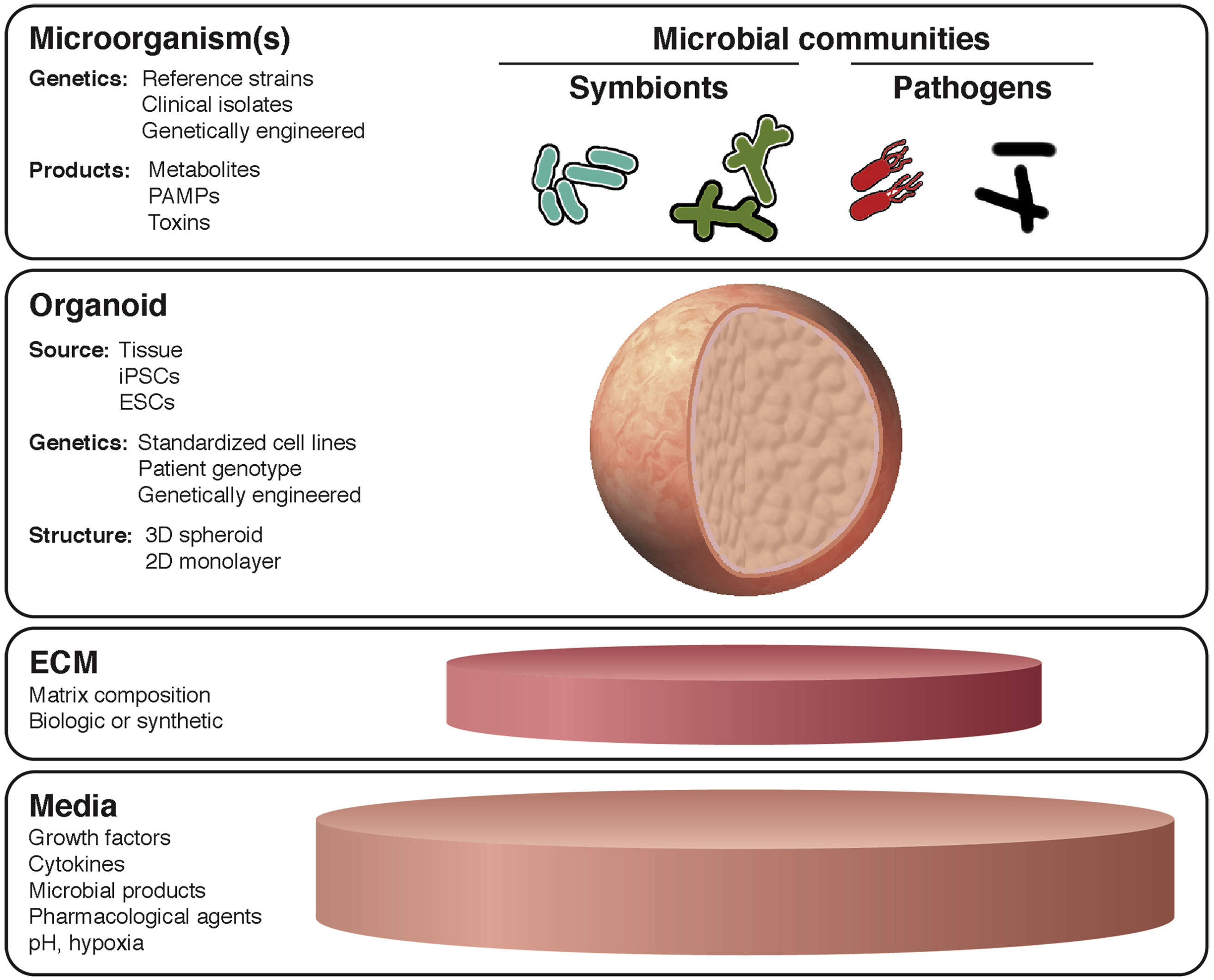
In recent years, increasing attention has been devoted to the concept that microorganisms play an integral role in human physiology and pathophysiology. Despite this, the molecular basis of host–pathogen and host–symbiont interactions in the human intestine remains poorly understood owing to the limited availability of human tissue, and the biological complexity of host–microbe interactions. Over the past decade, technological advances have enabled long-term culture of organotypic intestinal tissue derived from human subjects and from human pluripotent stem cells, and these in vitro culture systems already have shown the potential to inform our understanding significantly of host-microbe interactions. Gastrointestinal organoids represent a substantial advance in structural and functional complexity over traditional in vitro cell culture models of the human gastrointestinal epithelium while retaining much of the genetic and molecular tractability that makes in vitro experimentation so appealing. The opportunity to model epithelial barrier dynamics, cellular differentiation, and proliferation more accurately in specific intestinal segments and in tissue containing a proportional representation of the diverse epithelial subtypes found in the native gut greatly enhances the translational potential of organotypic gastrointestinal culture systems. By using these tools, researchers have uncovered novel aspects of host–pathogen and host–symbiont interactions with the intestinal epithelium. Application of these tools promises to reveal new insights into the pathogenesis of infectious disease, inflammation, cancer, and the role of microorganisms in intestinal development. This review summarizes research on the use of gastrointestinal organoids as a model of the host–microbe interface.
David R Hill, Jason R Spence
CMGH,
2017